Erectile dysfunction
Adult: Initially, 100 mg 15-30 min before sexual intercourse. Dose may be decreased to 50 mg or increased to a max of 200 mg, depending on response. Max dosing frequency: Once every 24 hr.
|
Indications and Dosage
Oral
Erectile dysfunction Adult: Initially, 100 mg 15-30 min before sexual intercourse. Dose may be decreased to 50 mg or increased to a max of 200 mg, depending on response. Max dosing frequency: Once every 24 hr.
|
||||
|
Special Patient Group
Patient receiving α-adrenergic blocking agent: Initially, 50 mg 15-30 min before sexual intercourse. Dose may be adjusted up to 200 mg, depending on response.
Patient receiving moderate CYP3A4 inhibitor (e.g. diltiazem, verapamil): Max: 50 mg once every 24 hr or 100 mg once every 48 hr. All doses are taken 15-30 min before sexual intercourse. |
||||
|
Renal Impairment
|
||||
|
Hepatic Impairment
Mild to moderate (Child-Pugh class A and B): Initiate w/ the lowest effective dose and adjust according to response. Severe (Child-Pugh class C): Contraindicated.
|
||||
|
Administration
tab: May be taken with or without food. Avoid grapefruit juice.
|
||||
|
Contraindications
Unstable angina, angina w/ sexual intercourse, CHF, resting hypotension (BP <90 mmHg) or HTN (BP >170/100 mmHg); recent (w/in the last 6 mth) MI, stroke, or life-threatening arrhythmia; other conditions wherein sexual activity is inadvisable, vision loss due to non-arteritic anterior ischaemic optic neuropathy (NAION), hereditary degenerative retinal disorders. Severe renal and hepatic (Child-Pugh class C) impairment. Concomitant use w/ any form of organic nitrate, guanylate cyclase stimulators, potent CYP3A4 inhibitors.
|
||||
|
Special Precautions
Patient w/ risk factors or pre-existing CV disease, left-ventricular outflow obstruction, active peptic ulcer, bleeding disorders, risk factors for priapism (e.g. patient w/ sickle cell anaemia, multiple myeloma, leukaemia), penile deformation. Mild to moderate hepatic impairment (Child-Pugh class A and B). Patients receiving α-adrenergic blockers or moderate CYP3A4 inhibitors.
|
||||
|
Adverse Reactions
Significant: CV risk, priapism, sudden vision and hearing loss, NAION, prolonged erection, hypersensitivity reactions.
Nervous: Headache, dizziness. CV: Flushing, ECG abnormality, transient sitting hypotension. GI: Viral gastroenteritis. Resp: Nasal congestion, nasopharyngitis, upper resp tract infection. Musculoskeletal: Back pain. Ophthalmologic: Impaired colour discrimination. Otic: Tinnitus. |
||||
|
Patient Counseling Information
This drug may cause dizziness and altered vision, if affected, do not drive or operate machinery.
|
||||
|
Monitoring Parameters
Assess CV status prior to therapy.
|
||||
|
Overdosage
Symptoms: Severe headache, flushing, nasopharyngitis, nasal congestion, back pain, dizziness. Management: Supportive treatment.
|
||||
|
Drug Interactions
Increased exposure w/ moderate CYP3A4 inhibitors (e.g. diltiazem, verapamil). Additive BP-lowering effect w/ α-adrenergic blocking agents (e.g. doxazosin, tamsulosin).
Potentially Fatal: May potentiate the hypotensive effect of any form of organic nitrate, guanylate cyclase stimulators (e.g. riociguat). Significantly increased systemic exposure w/ potent CYP3A4 inhibitors (e.g. ketoconazole, clarithromycin, ritonavir). |
||||
|
Food Interaction
Grapefruit juice may increases avanafil exposure. Enhanced hypotensive effect w/ alcohol.
|
||||
|
Action
Description:
Mechanism of Action: Avanafil inhibits phosphodiesterase type 5-mediated metabolism of cyclic guanosine monophosphate (cGMP) in the corpus cavernosum, resulting in increased cGMP levels which is responsible for smooth muscle relaxation and increased inflow of blood into the corpus cavernosum of the penis during sexual stimulation thereby producing an erection. It has no effect in the absence of sexual stimulation. Pharmacokinetics: Absorption: Rapidly absorbed from the GI tract. Time to peak plasma concentration: W/in 30-45 min. Distribution: Plasma protein binding: Approx 99%. Metabolism: Metabolised in the liver by CYP3A4 and to a minor extent by CYP2C isoenzymes into M4 (active) and M16 (inactive) metabolites. Excretion: Mainly via faeces (approx 62%, as metabolites); urine (approx 21%, as metabolites). Terminal elimination half-life: Approx 5-17 hr. |
||||
|
Chemical Structure
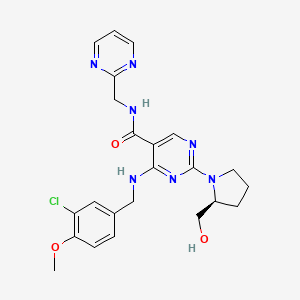 Source: National Center for Biotechnology Information. PubChem Database. Avanafil, CID=9869929, https://pubchem.ncbi.nlm.nih.gov/compound/Avanafil (accessed on Jan. 21, 2020) |
||||
|
Storage
Store between 20-25°C. Protect from light.
|
||||
|
ATC Classification
G04BE10 - avanafil ; Belongs to the class of drugs used in erectile dysfunction.
|
||||
|
References
Anon. Avanafil. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 01/08/2017. Buckingham R (ed). Avanafil. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 01/08/2017. Joint Formulary Committee. Avanafil. British National Formulary [online]. London. BMJ Group and Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 01/08/2017. McEvoy GK, Snow EK, Miller J et al (eds). Avanafil. AHFS Drug Information (AHFS DI) [online]. American Society of Health-System Pharmacists (ASHP). https://www.medicinescomplete.com. Accessed 01/08/2017. Stendra Tablet (Mist Pharmaceuticals, LLC). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 01/08/2017.
|