Ocular hypertension, Open-angle glaucoma
Adult: As monotherapy or adjunctive therapy to β-blockers or prostaglandin analogues: As 1% susp: Instil 1 drop into the conjunctival sac of the affected eye(s) 2 or 3 times daily.
|
Indications and Dosage
Ophthalmic
Ocular hypertension, Open-angle glaucoma Adult: As monotherapy or adjunctive therapy to β-blockers or prostaglandin analogues: As 1% susp: Instil 1 drop into the conjunctival sac of the affected eye(s) 2 or 3 times daily.
|
||||
|
Renal Impairment
|
||||
|
Contraindications
Hypersensitivity to brinzolamide or sulfonamides. Hyperchloraemic acidosis. Severe renal impairment (CrCl <30 mL/min).
|
||||
|
Special Precautions
Patient with compromised corneas (particularly with low endothelial cell count), pseudoexfoliative glaucoma, pigmentary glaucoma, risk of renal impairment, significant renal tubular abnormality. Not recommended for use in patients with narrow-angle glaucoma. Hepatic impairment. Pregnancy and lactation.
|
||||
|
Adverse Reactions
Eye disorders: Blurred vision, foreign body sensation in eyes, ocular hyperaemia, blepharitis, ocular discharge, ocular discomfort, ocular keratitis, ocular pain, ocular pruritus, keratoconjunctivitis, conjunctivitis, keratopathy, increased lacrimation, lid margin crusting or sticky sensation, dry eye, diplopia, eye fatigue.
Gastrointestinal disorders: Dysgeusia (bitter, sour, or unusual taste), nausea, diarrhoea, dry mouth, dyspepsia. General disorders and administration site conditions: Chest pain. Immune system disorders: Hypersensitivity. Nervous system disorders: Headache, dizziness, hypertonia. Respiratory, thoracic and mediastinal disorders: Rhinitis, pharyngitis, dyspnoea. Renal and urinary disorders: Renal pain. Skin and subcutaneous tissue disorders: Dermatitis, urticaria, alopecia. Potentially Fatal: Rarely, Stevens-Johnson syndrome, toxic epidermal necrolysis, fulminant hepatic necrosis, agranulocytosis, aplastic anaemia, and other blood dyscrasias. |
||||
|
Ophth: C
|
||||
|
Patient Counseling Information
This drug may cause temporary blurred vision or other visual disturbances, if affected, do not drive or operate machinery. Remove contact lenses before administration and reinsert them after 15 minutes.
|
||||
|
Monitoring Parameters
Perform ophthalmic exams and monitor IOP.
|
||||
|
Drug Interactions
Concurrent use with oral carbonic anhydrase inhibitors (e.g. acetazolamide, methazolamide) may lead to additive systemic effects. CYP3A4 inhibitors (e.g. ketoconazole, itraconazole, clotrimazole, ritonavir, troleandomycin) may inhibit the metabolism of brinzolamide. Concurrent use with high-dose salicylates may lead to toxicity.
|
||||
|
Action
Description:
Mechanism of Action: Brinzolamide is an inhibitor of carbonic anhydrase II (CAII), the main isoenzyme involved in aqueous humour secretion. It inhibits carbonic anhydrase in the ciliary processes of the eye, presumably by slowing bicarbonate formation and reducing Na and fluid transport, thus reducing aqueous humour secretion and subsequently decreasing intraocular pressure (IOP). Pharmacokinetics: Absorption: Absorbed into systemic circulation. Distribution: Accumulates in RBCs. Plasma protein binding: Approx 60%. Metabolism: Metabolised to N-desethyl brinzolamide, which also binds to carbonic anhydrase and accumulates in RBCs. Excretion: Mainly via urine (as unchanged drug and metabolites). |
||||
|
Chemical Structure
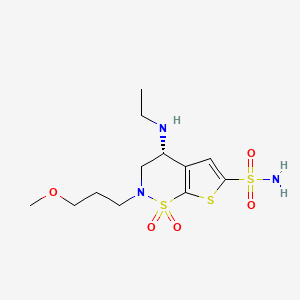 Source: National Center for Biotechnology Information. PubChem Compound Summary for CID 68844, Brinzolamide. https://pubchem.ncbi.nlm.nih.gov/compound/Brinzolamide. Accessed Oct. 23, 2023. |
||||
|
Storage
Store between 4-30°C.
|
||||
|
MIMS Class
|
||||
|
ATC Classification
S01EC04 - brinzolamide ; Belongs to the class of carbonic anhydrase inhibitors. Used in the treatment of glaucoma.
|
||||
|
References
Anon. Brinzolamide. AHFS Clinical Drug Information [online]. Bethesda, MD. American Society of Health-System Pharmacists, Inc. https://www.ahfscdi.com. Accessed 16/10/2023. Anon. Brinzolamide. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 04/10/2023. Azopt 10 mg/mL Eye Drops, Suspension (Novartis Corporation [Malaysia] Sdn. Bhd.). National Pharmaceutical Regulatory Agency - Ministry of Health Malaysia. https://www.npra.gov.my. Accessed 16/10/2023. Azopt Suspension/Drops (Novartis Pharmaceuticals Corporation). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed. Accessed 16/10/2023. Brinzolamide 10 mg/mL Eye Drops, Suspension (Pharmathen S.A.). MHRA. https://products.mhra.gov.uk. Accessed 04/10/2023. Brinzolamide Suspension/Drops (Actavis Pharma, Inc.). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed. Accessed 04/10/2023. Brinzolamide. Gold Standard Drug Database in ClinicalKey [online]. Elsevier Inc. https://www.clinicalkey.com. Accessed 04/10/2023. Buckingham R (ed). Brinzolamide. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 04/10/2023. Joint Formulary Committee. Brinzolamide. British National Formulary [online]. London. BMJ Group and Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 04/10/2023. Miro Healthcare Ltd. Brinzolamide Indoco Eye Drops data sheet 23 March 2023. Medsafe. http://www.medsafe.govt.nz. Accessed 04/10/2023.
|