Community-acquired pneumonia, Hospital-acquired pneumonia
Adult: 500 mg 8 hourly via infusion over 120 minutes.
|
Indications and Dosage
Intravenous
Community-acquired pneumonia, Hospital-acquired pneumonia Adult: 500 mg 8 hourly via infusion over 120 minutes.
|
||||||
|
Renal Impairment
ESRD (including haemodialysis): 250 mg 24 hourly.
|
||||||
|
Reconstitution
Add 10 mL of sterile water for inj or dextrose 5% solution to a vial containing 500 mg to provide a 50 mg/mL solution. Shake vigorously. Further dilute reconstituted solution in a 125 or 250 mL NaCl 0.9% solution, dextrose 5% in water or Lactated Ringer’s solution for inj.
|
||||||
|
Incompatibility
Incompatible with Ca-containing solutions (except Lactated Ringer’s solution for inj). Y-site: Acyclovir, amikacin, ciprofloxacin, caspofungin, acyclovir Na, amikacin sulfate, amiodarone hydrochloride, amphotericin b (colloidal), Ca gluconate, caspofungin acetate, ciprofloxacin, cisatracurium besylate, diazepam, diltiazem hydrochloride, diphenhydramine hydrochloride, dobutamine hydrochloride, dopamine hydrochloride, esomeprazole Na, famotidine, filgrastim, gentamicin sulfate, haloperidol lactate, hydromorphone hydrochloride, hydroxyzine hydrochloride, insulin human regular, insulin lispro, labetalol hydrochloride, levofloxacin, lidocaine hydrochloride, Mg sulfate, meperidine hydrochloride, metoclopramide hydrochloride, midazolam hydrochloride, milrinone lactate, morphine sulfate, moxifloxacin hydrochloride, ondansetron hydrochloride, pantoprazole sodium, K phosphates, promethazine hydrochloride, remifentanil hydrochloride, Na phosphates, tobramycin sulfate.
|
||||||
|
Contraindications
Hypersensitivity to cephalosporins, immediate and severe hypersensitivity to other β-lactam antibacterials (e.g. penicillins, carbapenems).
|
||||||
|
Special Precautions
Patient with history of non-severe hypersensitivity to other β-lactam antibacterials, seizure disorder, ventilator-associated pneumonia (VAP), immunocompromised. Renal impairment. Pregnancy and lactation.
|
||||||
|
Adverse Reactions
Significant: Thrombocytopenia, agranulocytosis, convulsion, agitation, renal failure, super infection with non-susceptible organisms.
Gastrointestinal disorders: Nausea, vomiting, diarrhoea, abdominal pain, dyspepsia. Hepatobiliary disorders: Increased hepatic enzymes. Infections and infestations: Fungal infection. Injury, poisoning and procedural complications: Infusion site reactions. Metabolism and nutrition disorders: Hyponatraemia, hypokalaemia. Nervous system disorders: Dysgeusia, headache, dizziness, somnolence. Psychiatric disorders: Insomnia, agitation. Respiratory, thoracic and mediastinal disorders: Dyspnoea. Skin and subcutaneous tissue disorders: Rash, pruritus. Potentially Fatal: Hypersensitivity reactions, Clostridium difficile-associated diarrhoea. |
||||||
|
Patient Counseling Information
This drug may cause dizziness, if affected, do not drive or operate machinery.
|
||||||
|
Monitoring Parameters
Monitor renal function and for signs of hypersensitivity reactions during first dose.
|
||||||
|
Drug Interactions
May increase serum concentration with statin, glyburide, bosentan.
|
||||||
|
Lab Interference
May give false positive Coombs’ test result. False-positive reaction in urinary glucose test using copper reduction technique. May interfere with serum creatinine to alkaline picrate and may give falsely elevated creatinine measurements.
|
||||||
|
Action
Description:
Mechanism of Action: Ceftobiprole is a 5th generation cephalosporin which exerts bactericidal activity by binding to penicillin-binding proteins (PBP) 2a in methicillin-resistant Staphylococcus aureus strains (MRSA) thus inhibiting cell wall biosynthesis thereby causing bacterial cell death. It also binds to PBP2b in Streptococcus pneumoniae (penicillin-intermediate), PBP2x in S. pneumoniae (penicillin resistant), and PBP5 in Enterococcus faecalis. Pharmacokinetics: Absorption: Time to peak plasma concentration: 29.2-33 hours. Distribution: Volume of distribution: 18 L. Plasma protein binding: 16%. Excretion: Mainly via urine (approx 89%). Elimination half-life: Approx 3 hours. |
||||||
|
Chemical Structure
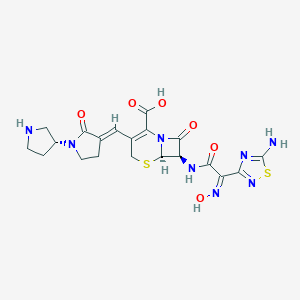 Source: National Center for Biotechnology Information. PubChem Database. Ceftobiprole, CID=135413542, https://pubchem.ncbi.nlm.nih.gov/compound/Ceftobiprole (accessed on Jan. 21, 2020) |
||||||
|
Storage
Store between 2-8°C. Protect from light.
|
||||||
|
MIMS Class
|
||||||
|
ATC Classification
J01DI01 - ceftobiprole medocaril ; Belongs to the class of other cephalosporins and penems. Used in the systemic treatment of infections.
|
||||||
|
References
Buckingham R (ed). Ceftobiprole Medocaril. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 08/03/2018. Joint Formulary Committee. Ceftobiprole. British National Formulary [online]. London. BMJ Group and Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 08/03/2018.
|