Skin and skin structure infections
Adult: 300 mg every 12 hours, via infusion over 60 minutes for 5-14 days.
Oral
Skin and skin structure infections
Adult: 450 mg every 12 hours for 5-14 days.
|
Indications and Dosage
Intravenous
Skin and skin structure infections Adult: 300 mg every 12 hours, via infusion over 60 minutes for 5-14 days. Oral Skin and skin structure infections Adult: 450 mg every 12 hours for 5-14 days.
|
|
Renal Impairment
Oral
Estimated GFR (eGFR) <15 mL/min/1.73m2, ESRD, patients on haemodialysis: Not recommended. IV eGFR <15 mL/min/1.73m2, ESRD, patients on haemodialysis: Not recommended. eGFR 15-29 mL/min/1.73m2: 200 mg every 12 hours. |
|
Administration
May be taken with or without food. Take at least 2 hr before or 6 hr after antacids containing Mg or Al, sucralfate, & supplements containing Zn or Fe.
|
|
Reconstitution
IV Infusion: Reconstitute vials labelled as containing 300 mg with 10.5 mL NaCl 0.9% or dextrose 5% in water to provide a solution containing 25 mg/mL. Shake the vial vigorously to completely dissolve. Further dilute with NaCl 0.9% or dextrose 5% inj to a total volume of 250 mL with a final concentration of 1.2 mg/mL.
|
|
Incompatibility
Incompatible with solutions containing multivalent cations (e.g. Ca, Mg).
|
|
Contraindications
Hypersensitivity to delafloxacin or other fluoroquinolones. Patient with known history of tendon disorders (e.g. tendinitis or tendon rupture), peripheral neuropathy, myasthenia gravis. Concomitant administration with live vaccines.
|
|
Special Precautions
Patient with known or suspected CNS disorder (e.g. severe cerebral arteriosclerosis, epilepsy), risk factors that may predispose to seizures, risk factors for tendon rupture (e.g. solid organ transplant recipients, history of rheumatoid arthritis). Renal impairment. Elderly.
|
|
Adverse Reactions
Significant: Hyperglycaemia, hypoglycaemia leading to coma, superinfection (e.g. Clostridium difficile-associated diarrhoea (CDAD) or pseudomembranous colitis), irreversible tendinitis or tendon rupture, peripheral neuropathy, CNS effects (e.g. seizures, increased, intracranial pressure); toxic psychosis (e.g. disturbances in attention, disorientation, agitation, nervousness, memory impairment, depression, suicidality, delirium).
Gastrointestinal disorders: Nausea, vomiting, diarrhoea. Investigations: Elevated transaminases. Nervous system disorders: Headache. Potentially Fatal: Hypersensitivity reactions (e.g. anaphylaxis). |
|
Patient Counseling Information
This drug may cause dizziness and lightheadedness, if affected, do not drive or operate machinery.
|
|
Monitoring Parameters
Monitor serum creatinine, GFR levels, CBC, LFT, blood glucose levels. Monitor for signs and symptoms of hypersensitivity reactions, tendon problems, and infection. Perform culture and sensitivity tests prior to initiating therapy, test for C. difficile if diarrhoea develops.
|
|
Drug Interactions
May enhance hypoglycaemic effect of oral hypoglycaemic agents. Decreased absorption by forming chelates with antacids containing Al, Mg.
Potentially Fatal: May diminish therapeutic effect of live vaccines. |
|
Action
Description:
Mechanism of Action: Delafloxacin is a fluoroquinolone anti-infective agent, which inhibits bacterial topoisomerase IV and DNA gyrase (topoisomerase II) enzymes which are essential for bacterial DNA replication, transcription, repair and recombination thereby impeding bacterial growth. Pharmacokinetics: Absorption: Bioavailability: 58.8%. Time to peak plasma concentration: Approx 1 hour. Distribution: Plasma protein binding: Approx 84%, mainly to albumin. Metabolism: Primarily metabolised via glucuronidation by uridine diphosphate-glucuronosyltransferase (UGT) 1A1, 1A3, and 2B15. Excretion: Via urine (50% (oral), 65% (IV), as unchanged drug); faeces (48% (oral), 28% (IV), as unchanged drug. Elimination half-life: 4.2-8.5 hours (oral); 3.7 hours (IV). |
|
Chemical Structure
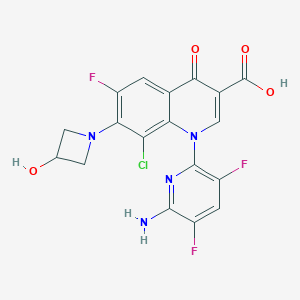 Source: National Center for Biotechnology Information. PubChem Database. Delafloxacin, CID=487101, https://pubchem.ncbi.nlm.nih.gov/compound/Delafloxacin (accessed on Jan. 20, 2020) |
|
Storage
Store between 20-25°C. Reconstituted solution: Store between 20-25°C or refrigerate between 2-8°C for up to 24 hours. Do not freeze.
|
|
MIMS Class
|
|
ATC Classification
J01MA23 - delafloxacin ; Belongs to the class of fluoroquinolones. Used in the systemic treatment of infections.
|
|
References
Anon. Delafloxacin. AHFS Clinical Drug Information [online]. Bethesda, MD. American Society of Health-System Pharmacists, Inc. https://www.ahfscdi.com/. Accessed 03/09/2018 . Anon. Delafloxacin. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 03/09/2018 . Baxdela (Melinta Therapeutics, Inc.). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 03/09/2018 . Baxdela (Melinta Therapeutics, Inc.). U.S. FDA. https://www.fda.gov/. Accessed 03/09/2018 . Buckingham R (ed). Delafloxacin. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com . Accessed 03/09/2018 .
|