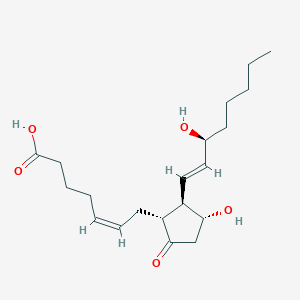
Pregnancy termination in the 2nd trimester
Adult: Instill 1 mL via suitable foley catheter followed by 1-2 mL at 2 hr intervals according to patient's response.
Intracervical
Cervical priming, induction and augmentation of labour
Adult: As cervical gel: Insert 0.5 mg into the cervical canal, may be repeated at 6 hr interval, if needed. Max: 1.5 mg/24 hr.
Intravenous
Pregnancy termination in the 2nd trimester
Adult: 2.5 mcg/min via infusion over 30 min, then maintain or increase to 5 mcg/min. Maintain for at least 4 hr before increasing further.
Vaginal
Labour induction
Adult: As vag gel: 1 mg (or 2 mg in primigravid patient w/ unfavourable induction features) inserted into the posterior fornix, followed by 1-2 mg after 6 hr, if needed. Max: 3 mg (or 4 mg in unfavourable primigravid patient) per course. As vag tab: 3 mg inserted high into the posterior fornix, followed by a further 3 mg after 6-8 hr, if necessary. Max: 6 mg per course.
Vaginal
Pregnancy termination in the 2nd trimester
Adult: As vag supp: Insert 1 supp (20 mg) high into the posterior fornix, repeat at 3-5 hr interval until abortion occurs, for up to 2 days.
Vaginal
Cervical priming, induction and augmentation of labour
Adult: As vag device or insert delivering 0.3 mg/hr: Administer 1 vag device/insert (10 mg) high into posterior fornix and remove when cervical ripening is complete, or after 12-24 hr (depending on product) if cervical ripening is insufficient.




 Sign Out
Sign Out