Hypersensitivity reactions
Adult: Up to 25 mg 4-6 hrly. Max: 150 mg daily.
Oral
Short-term management of insomnia
Adult: 25 mg given 30 min before bedtime.
|
Indications and Dosage
Oral
Hypersensitivity reactions Adult: Up to 25 mg 4-6 hrly. Max: 150 mg daily. Oral Short-term management of insomnia Adult: 25 mg given 30 min before bedtime.
|
|
Administration
Should be taken with food. Take w/ food or milk.
|
|
Contraindications
Lactation.
|
|
Special Precautions
Patient w/ increased intraocular pressure/glaucoma, urinary retention, prostate enlargement, pyloroduodenal obstruction, thyroid dysfunction, resp disease, epilepsy or severe CV disorder (e.g. HTN, ischaemic heart disease). Pregnancy.
|
|
Adverse Reactions
CNS depression, stimulation (e.g. insomnia, nervousness, euphoria, irritability, tremors, nightmares, hallucinations, convulsions); headache, lack of coordination, dizziness, psychomotor impairment, constipation, nausea, vomiting, diarrhoea, increased gastric reflux, epigastric pain, thickened resp tract secretions, dry mouth, palpitations, arrhythmias, blurred vision, urinary retention, blood disorders, hypotension, tinnitus, paraesthesia, hypersensitivity reactions.
|
|
PO: B (FDA Pregnancy Category A applies to doxylamine succinate w/ pyridoxine hydrochloride.)
|
|
Patient Counseling Information
This drug may cause drowsiness, if affected, do not drive or operate machinery.
|
|
Overdosage
Symptoms: Dry mouth, fixed and dilated pupils, flushing, GI symptoms, insomnia, nervousness, euphoria, irritability, tremors, nightmares, hallucinations, CNS depression or stimulation (esp in child), impaired consciousness, seizures, tachycardia, mydriasis, psychosis, rhabdomyolysis, coma and cardiorespiratory arrest. Management: Supportive and symptomatic treatment. Ensure adequate ventilation and hydration. Perform gastric lavage or administer activated charcoal w/in 60 min of ingestion. Whole bowel irrigation w/ polyethylene glycol electrolyte may be given to patients w/ extremely large ingestions.
|
|
Drug Interactions
Additive effect when administered w/ other CNS depressants (e.g. opioids analgesics, neuroleptics, hypnotics, other psychotherapeutic drugs). Antimuscarinic effects may be enhanced by MAOIs. May decrease emetic response to apomorphine. Additive antimuscarinic effects w/ other antimuscarinic drugs (e.g. atropine, TCAs).
|
|
Food Interaction
May enhance the CNS depressant effect of alcohol.
|
|
Lab Interference
May cause false-positive reaction in urine detection of methadone. May suppress positive skin test results.
|
|
Action
Description:
Mechanism of Action: Doxylamine, an ethanolamine derivative antihistamine, competitively blocks histamine at H1-receptor sites. It also diminishes vestibular stimulation and depresses labyrinthine function through its central anticholinergic activity. Duration: 6-8 hr. Pharmacokinetics: Absorption: Absorbed easily from the GI tract. Time to peak plasma concentration: 2-4 hr. Distribution: Enters breast milk (small amount). Volume of distribution: 2.5 L/kg. Metabolism: Metabolised hepatically via N-dealkylation to form metabolites. Excretion: Via urine, 60% as unchanged drug. Elimination half-life: 10-12 hr. |
|
Chemical Structure
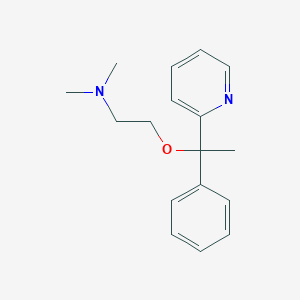 Source: National Center for Biotechnology Information. PubChem Database. Doxylamine, CID=3162, https://pubchem.ncbi.nlm.nih.gov/compound/Doxylamine (accessed on Jan. 21, 2020) |
|
Storage
Store between 15-30°C.
|
|
MIMS Class
|
|
ATC Classification
R06AA09 - doxylamine ; Belongs to the class of aminoalkyl ethers used as systemic antihistamines.
|
|
References
Anon. Doxylamine . Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 18/10/2016. Buckingham R (ed). Doxylamine Succinate. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com . Accessed 18/10/2016. McEvoy GK, Snow EK, Miller J et al (eds). Doxylamine Succinate. AHFS Drug Information (AHFS DI) [online]. American Society of Health-System Pharmacists (ASHP). https://www.medicinescomplete.com. Accessed 18/10/2016. Nighttime Sleep Aid (Wal-Mart Stores Inc). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 18/10/2016. Wilson Consumer Products. Dozile Capsules data sheet 16 July 2008. Medsafe. http://www.medsafe.govt.nz/. Accessed 18/10/2016.
|