Clostridium difficile-associated diarrhoea
Adult: 200 mg bid for 10 days.
|
Indications and Dosage
Oral
Clostridium difficile-associated diarrhoea Adult: 200 mg bid for 10 days.
|
|
Administration
May be taken with or without food.
|
|
Contraindications
Hypersensitivity.
|
|
Special Precautions
Patient w/ severe C. difficile infection, inflammatory bowel disease (e.g. ulcerative colitis or Crohn’s disease, toxic megacolon), known macrolide allergy. Not indicated for systemic infections. Moderate to severe hepatic and severe renal impairment. Pregnancy and lactation.
|
|
Adverse Reactions
Significant: Hypersensitivity reactions (e.g. dyspnoea, pruritus, rash, angioedema of mouth, face and throat).
Nervous: Dizziness, headache. GI: Nausea, GI haemorrhage, abdominal pain, vomiting, abdominal distention, tenderness, dyspepsia, dysphagia, flatulence, intestinal obstruction, megacolon, anorexia, dysgeusia, constipation. Hepatic: Increased ALT. Haematologic: Anaemia, neutropenia. |
|
Monitoring Parameters
Monitor for signs and symptoms of hypersensitivity.
|
|
Drug Interactions
May diminish the therapeutic effect of Na picosulfate, lactobacillus and estriol. May increase serum concentration of mizolastine. Increased plasma concentration w/ P-gp inhibitor (e.g. ciclosporin).
|
|
Action
Description:
Mechanism of Action: Fidaxomicin is a macrocyclic antibiotic. It inhibits Clostridium difficile through inhibition of RNA synthesis by binding to RNA polymerase sigma subunit. Pharmacokinetics: Absorption: Poorly absorbed from the GI tract. Time to peak plasma concentration: 1.75 hr. Distribution: Largely confined to the GI tract. Metabolism: Undergoes intestinal metabolism via hydrolysis to less active metabolite (OP-1118). Excretion: Mainly via faeces (>92% as unchanged drug and metabolites); urine (<1% as metabolite). Elimination half-life: Approx 12 hr (fidaxomicin); approx 11 hr (OP-1118). |
|
Chemical Structure
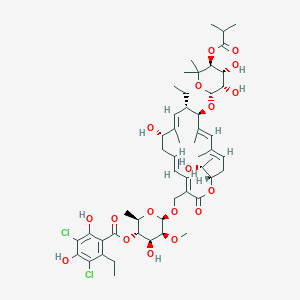 Source: National Center for Biotechnology Information. PubChem Database. Dificid, CID=10034073, https://pubchem.ncbi.nlm.nih.gov/compound/Dificid (accessed on Jan. 21, 2020) |
|
Storage
Store between 20-25°C.
|
|
MIMS Class
|
|
References
Anon. Fidaxomicin. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 05/09/2017. Buckingham R (ed). Fidaxomicin. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 05/09/2017. Dificid Tablet, Film Coated (Merck Sharp & Dohme Corp.). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 05/09/2017. Joint Formulary Committee. Fidaxomicin. British National Formulary [online]. London. BMJ Group and Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 05/09/2017. McEvoy GK, Snow EK, Miller J et al (eds). Fidaxomicin. AHFS Drug Information (AHFS DI) [online]. American Society of Health-System Pharmacists (ASHP). https://www.medicinescomplete.com. Accessed 05/09/2017.
|