Relapsing remitting multiple sclerosis
Adult: 0.5 mg once daily.
|
Indications and Dosage
Oral
Relapsing remitting multiple sclerosis Adult: 0.5 mg once daily.
|
|
Hepatic Impairment
Severe: Contraindicated.
|
|
Administration
May be taken with or without food.
|
|
Contraindications
Known immunodeficiency syndrome and active malignancies; severe active or chronic infections (e.g. hepatitis, TB). Patient w/ increased risk for opportunistic infections, including immunocompromised patients (e.g. receiving immunosuppressive therapies or immunocompromised by prior therapies). Recent (i.e. w/in 6 mth) MI, unstable angina, stroke, transient ischaemic attack, decompensated heart failure requiring hospitalisation or class III/IV heart failure. History or presence of Mobitz type II 2nd- or 3rd-degree AV block or sick sinus syndrome (unless w/ functioning pacemaker); baseline QTc interval ≥500 msec. Severe hepatic impairment. Lactation. Concurrent class Ia or III antiarrhythmic agents.
|
|
Special Precautions
Patient w/ history of symptomatic bradycardia or recurrent syncope; CV diseases e.g. ischaemic heart disease, CHF, cerebrovascular disease, uncontrolled HTN; DM. Mild to moderate hepatic and severe renal impairment. Pregnancy.
|
|
Adverse Reactions
Back pain, cough, diarrhoea, headache, influenza, elevated liver enzymes; reduction in heart rate, hypotension, dizziness, fatigue, palpitations, chest pain, transient AV conduction, complete AV block, macular oedema, dyspnoea, HTN. Rarely, posterior reversible encephalopathy, causing altered mental status, visual disturbances and seizures.
Potentially Fatal: Progressive multifocal leukoencephalopathy (PML), disseminated herpes infection (e.g. disseminated primary herpes zoster, herpes simplex encephalitis). |
|
Monitoring Parameters
Observe for signs and symptoms of bradycardia, hrly measurements of heart rate and BP during treatment; baseline ECG and after 6 hr. Monitor for signs and symptoms of infection during therapy and for 2 mth thereafter. Perform ophth evaluation before starting and routinely (approx 3-4 mth) after treatment. Monitor hepatic transaminases before treatment, then 3 mthly for 1 yr, then periodically thereafter. Monitor FBC before treatment, at 3 mth, then at least yrly thereafter and if w/ signs of infection.
|
|
Drug Interactions
Increased risk of immunosuppression w/ antineoplastics, immunosuppressants and immunomodulators. Increased risk of bradycardia w/ drugs that slow the heart rate or AV conductions (e.g. β-blockers, Ca-channel blockers). Increased blood concentrations w/ ketoconazole. Strong CYP3A4 inducers and inhibitors may affect metabolism of fingolimod. Reduces immune response to vaccination.
Potentially Fatal: Enhanced arrhythmogenic effect of class Ia or III antiarrhythmic agents. |
|
Action
Description:
Mechanism of Action: Fingolimod is a sphingosine 1-phosphate receptor modulator. It is metabolised by sphingosine kinase to the active metabolite fingolimod phosphate which binds to sphingosine 1-phosphate receptors 1, 3, 4, and 5. Fingolimod phosphate blocks the lymphocytes' ability to emerge from lymph nodes, thus, decreasing the amount of lymphocytes available to the CNS, which reduces central inflammation. Pharmacokinetics: Absorption: Slowly absorbed from the GI tract. Bioavailability: Approx 93%. Time to peak plasma concentration: Approx 12-16 hr. Distribution: Highly distributed into RBCs (86%), extensively distributed into body tissues. Plasma protein binding: >99%. Volume of distribution: Approx 1,200 L. Metabolism: Undergoes reversible stereoselective phosphorylation to the pharmacologically active (S)-enantiomer, fingolimod phosphate. It is eliminated via oxidative metabolism mainly by CYP4F2 isoenzyme, and subsequent fatty acid-like degradation to inactive metabolites. Excretion: Via urine (approx 81%) as inactive metabolites; faeces (<2.5%) as unchanged drug and active metabolite. Terminal half-life: Approx 6-9 days. |
|
Chemical Structure
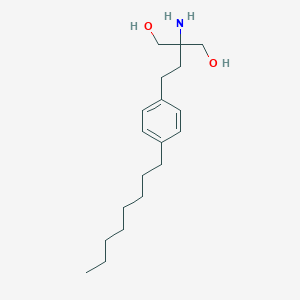 Source: National Center for Biotechnology Information. PubChem Database. Fingolimod, CID=107970, https://pubchem.ncbi.nlm.nih.gov/compound/Fingolimod (accessed on Jan. 21, 2020) |
|
Storage
Store at 25°C. Protect from moisture.
|
|
MIMS Class
|
|
ATC Classification
L04AA27 - fingolimod ; Belongs to the class of selective immunosuppressive agents. Used to induce immunosuppression.
|
|
References
Anon. Fingolimod. Lexicomp Online. Hudson, Ohio. Wolters Kluwer Clinical Drug Information, Inc. https://online.lexi.com. Accessed 11/03/2016. Buckingham R (ed). Fingolimod. Martindale: The Complete Drug Reference [online]. London. Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 11/03/2016. Gilenya 0.5 mg Hard Capsules (Novartis Pharmaceuticals UK Ltd). eMC. https://www.medicines.org.uk/emc/. Accessed 11/03/2016. Gilenya Capsule (Novartis Pharmaceuticals Corporation). DailyMed. Source: U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/. Accessed 11/03/2016. Joint Formulary Committee. Fingolimod. British National Formulary [online]. London. BMJ Group and Pharmaceutical Press. https://www.medicinescomplete.com. Accessed 11/03/2016. McEvoy GK, Snow EK, Miller J et al (eds). Fingolimod Hydrochloride. AHFS Drug Information (AHFS DI) [online]. American Society of Health-System Pharmacists (ASHP). https://www.medicinescomplete.com. Accessed 11/03/2016.
|